Gene therapists ask to be released from the RAC
March 11, 2012 | Source: Science
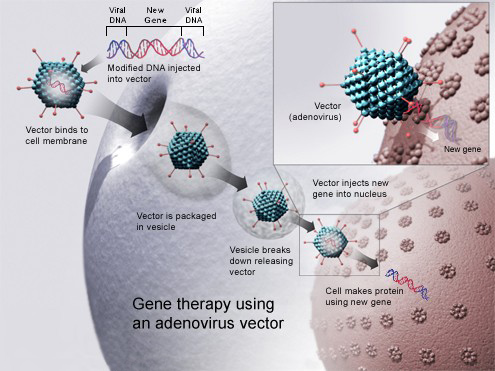
After 20 years of experience, more than 1000 U.S. trials, and a growing number of gene therapy successes in the clinic, some researchers say it’s time to end the Recombinant DNA Advisory Committee (RAC) review of gene therapy protocols.
One big reason: the proposals are already reviewed by the FDA and institutional ethics and biosafety boards, which now have plenty of experience with the field.
“Gene therapy is overregulated to the point where it’s crippling progress,” says Xandra Breakefield of Massachusetts General Hospital in Charlestown, president-elect of the American Society of Gene and Cell Therapy (ASGCT).