Controlling pain by optogenetic stimulation of the brain’s pain center
February 27, 2015
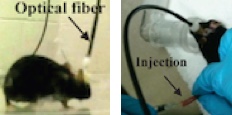
Pain-reduction experiment setup: (left) optical fiber mounted via cannula in mouse brain; (right) saline or Formalin injection in hind paw (credit: Ling Gu et al./PLoS ONE)
A small area of the brain called the anterior cingulate cortex (ACC) in the thalamus can be optically stimulated to control pain, University of Texas at Arlington scientists have found.
The researchers used optogenetic stimulation with a blue laser to control pain sensation in a mouse, created by a chemical irritant (formalin) and mechanical pain, such as that experienced following a pinprick or pinch.
“Our results clearly demonstrate, for the first time, that optogenetic stimulation of inhibitory neurons in ACC leads to decreased neuronal activity and a dramatic reduction of pain behavior,”said Samarendra Mohanty, an assistant professor of physics who leads the Biophysics and Physiology Lab in the UT Arlington College of Science and co-author on an open-access paper published online Wednesday Feb. 25 by the journal PLOS ONE.
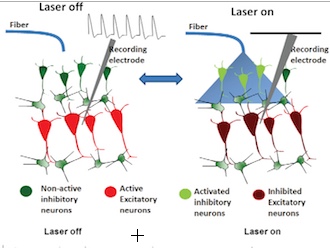
With laser off (left), pain-associated neurons in the ACC area of the thalamus showed activity when a pain-inducing stimulus was applied to a mouse paw; with laser on (right), no neural activity was detected (credit: Ling Gu et al./PLoS ONE)
Other researchers have previously experimented with using electrodes to stimulate inhibitory neurons (to reduce pain) in the ACC, but such stimulation lacks specificity and leads to activation of both excitatory and inhibitory neurons, he said.
Mohanty suggested that the results could lead to increased understanding of pain pathways and strategies for managing chronic pain, which often leads to severe impairment of normal psychological and physical functions.
However, “while reducing the sensation for chronic pain by optical stimulation, we still want to sense certain types of pain because they tell us to move our hands or legs away from something that is too hot or that might otherwise hurt us if we get too close,” Mohanty noted.
The team now plans to carry out localized non-invasive stimulation of small brain regions such as the ACC to better understand and control pain.
UT Dallas and University of Kentucky researchers also participated in the study. Mohanty’s lab is currently supported by a $384,269 two-year grant from the National Institutes of Health National Institute of Neurological Disorders and Strokes.
Abstract of Pain Inhibition by Optogenetic Activation of Specific Anterior Cingulate Cortical Neurons
Cumulative evidence from both humans and animals suggests that the anterior cingulate cortex (ACC) is important for pain-related perception, and thus a likely target for pain relief therapy. However, use of existing electrode based ACC stimulation has not significantly reduced pain, at least in part due to the lack of specificity and likely co-activation of both excitatory and inhibitory neurons. Herein, we report a dramatic reduction of pain behavior in transgenic mice by optogenetic stimulation of the inhibitory neural circuitry of the ACC expressing channelrhodopsin-2. Electrophysiological measurements confirmed that stimulation of ACC inhibitory neurons is associated with decreased neural activity in the ACC. Further, a distinct optogenetic stimulation intensity and frequency-dependent inhibition of spiking activity in the ACC was observed. Moreover, we confirmed specific electrophysiological responses from different neuronal units in the thalamus, in response to particular types of painful stimuli (i,e., formalin injection, pinch), which we found to be modulated by optogenetic control of the ACC inhibitory neurons. These results underscore the inhibition of the ACC as a clinical alternative in inhibiting chronic pain, and leads to a better understanding of the pain processing circuitry of the cingulate cortex.
