How the presence of water changes the structure of an antibiotic
April 20, 2012
EPFL chemists have shown how the three-dimensional shape of an antibiotic changes when it is in an aqueous environment. This could lead to a better understanding of how drugs interact with biological molecules.
Like a key inserted into a lock, the molecules in drugs bind with and act upon biomolecules. The more precisely we know these molecules’ three-dimensional structure, the better we will understand how their active components work. But water isn’t just a neutral environment; it can also interact with the molecules and change their structure.
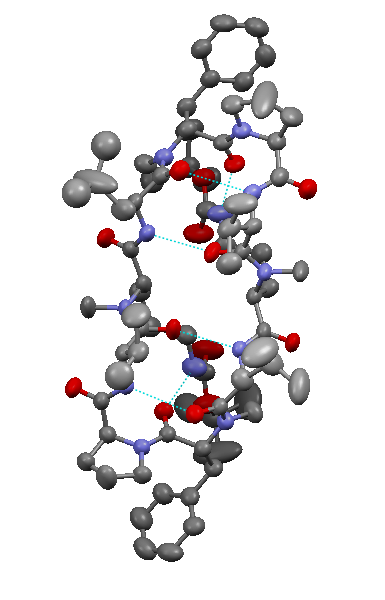
Scientists from EPFL’s Laboratory of Molecular Physical Chemistry have shown how the 3D structure of gramicidin — a natural antibiotic — changes depending on the number of water molecules surrounding it. Their research could lead to progress in designing new drugs that could be used against diseases such as epilepsy, cancer and Alzheimer’s disease.
To determine the structure of a molecule, chemists measure its spectrum, or the wavelengths of light that it absorbs when illuminated by a laser. This spectral signature allows them to predict the 3D structure of the molecule. Ideally, each spectrum corresponds to a particular structure.
But at room temperature, large molecules like gramicidin — made up of 176 atoms — are victims of a parasitic quantum effect known as inhomogeneous thermal broadening. This interference, associated with the thermal agitation of the atoms in the molecule, makes it impossible to get a precise enough measurement to be able to identify a unique molecular structure.
The chemists had to reduce the effects of these perturbations. To do this, they trapped the molecules in a specially designed device and then quickly cooled the molecules to about 10 degrees Kelvin. At this extremely cold temperature — just ten degrees above absolute zero — the parasitic quantum effects diminish and measurements can be made. Finally, they added water to the antibiotic, molecule by molecule, and observed that its structure changed as a function of the quantity of water molecules added. They were able to make measurements by adding up to fifty molecules of water at a time.
New drugs
This research promised to have an impact on both fundamental research and the pharmaceutical arena. “Understanding how drug molecules change shape when they are dissolved in water is a crucial point,” said senior scientist Oleg Boyarkine. “This discovery will make it possible to design new, more effective drugs using computers.”
Drugs developed to combat Alzheimer’s disease and epilepsy bind with proteins in the brain at specific points, and block disease progression. The goal of future research will be to determine the specific binding points between a drug and a protein, as well as their 3D structures in the presence of water. Understanding these structures will thus open up avenues for developing new drugs.
Ref.: Natalia S. Nagornova, Thomas R. Rizzo and Oleg V. Boyarkin, Interplay of Intra- and Intermolecular H-Bonding in a Progressively Solvated Macrocyclic Peptide, Science, 2012 [DOI: 10.1126/science.1218709]