MIT nanosystem delivers precise amounts of drugs directly to a tiny spot in the brain
January 28, 2018

MIT’s miniaturized system can deliver multiple drugs to precise locations in the brain, also monitor and control neural activity (credit: MIT)
MIT researchers have developed a miniaturized system that can deliver tiny quantities of medicine to targeted brain regions as small as 1 cubic millimeter, with precise control over how much drug is given. The goal is to treat diseases that affect specific brain circuits without interfering with the normal functions of the rest of the brain.*
“We believe this tiny microfabricated device could have tremendous impact in understanding brain diseases, as well as providing new ways of delivering biopharmaceuticals and performing biosensing in the brain,” says Robert Langer, the David H. Koch Institute Professor at MIT and one of the senior authors of an open-access paper that appears in the Jan. 24 issue of Science Translational Medicine.**
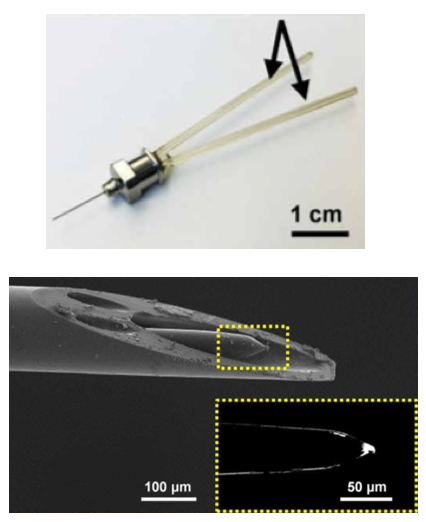
Miniaturized neural drug delivery system (MiNDS). Top: Miniaturized delivery needle with multiple fluidic channels for delivering different drugs. Bottom: scanning electron microscope image of cannula tip for delivering a drug or optogenetic light (to stimulate neurons) and a tungsten electrode (yellow dotted area — magnified view in inset) for detecting neural activity. (credit: Dagdeviren et al., Sci. Transl. Med., adapted by KurzweilAI)
The researchers used state-of-the-art microfabrication techniques to construct cannulas (thin tubes) with diameters of about 30 micrometers (width of a fine human hair) and lengths up to 10 centimeters. These cannulas are contained within a stainless steel needle with a diameter of about 150 micrometers. Inside the cannulas are small pumps that can deliver tiny doses (hundreds of nanoliters***) deep into the brains of rats — with very precise control over how much drug is given and where it goes.
In one experiment, they delivered a drug called muscimol to a rat brain region called the substantia nigra, which is located deep within the brain and helps to control movement. Previous studies have shown that muscimol induces symptoms similar to those seen in Parkinson’s disease. The researchers were able to stimulate the rats to continually turn in a clockwise direction. They also could also halt the Parkinsonian behavior by delivering a dose of saline through a different channel to wash the drug away.
“Since the device can be customizable, in the future we can have different channels for different chemicals, or for light, to target tumors or neurological disorders such as Parkinson’s disease or Alzheimer’s,” says Canan Dagdeviren, the LG Electronics Career Development Assistant Professor of Media Arts and Sciences and the lead author of the paper.
This device could also make it easier to deliver potential new treatments for behavioral neurological disorders such as addiction or obsessive compulsive disorder. (These may be caused by specific disruptions in how different parts of the brain communicate with each other.)
Measuring drug response
The researchers also showed that they could incorporate an electrode into the tip of the cannula, which can be used to monitor how neurons’ electrical activity changes after drug treatment. They are now working on adapting the device so it can also be used to measure chemical or mechanical changes that occur in the brain following drug treatment.
The cannulas can be fabricated in nearly any length or thickness, making it possible to adapt them for use in brains of different sizes, including the human brain, the researchers say.
“This study provides proof-of-concept experiments, in large animal models, that a small, miniaturized device can be safely implanted in the brain and provide miniaturized control of the electrical activity and function of single neurons or small groups of neurons. The impact of this could be significant in focal diseases of the brain, such as Parkinson’s disease,” says Antonio Chiocca, neurosurgeon-in-chief and chairman of the Department of Neurosurgery at Brigham and Women’s Hospital, who was not involved in the research.
The research was funded by the National Institutes of Health and the National Institute of Biomedical Imaging and Bioengineering.
* To treat brain disorders, drugs (such as l-dopa, a dopamine precursor used to treat Parkinson’s disease, and Prozac, used to boost serotonin levels in patients with depression) often interact with brain chemicals called neurotransmitters (or the cell receptors interact with neurotransmitters) — creating side effects throughout the brain.
** Michael Cima, the David H. Koch Professor of Engineering in the Department of Materials Science and Engineering and a member of MIT’s Koch Institute for Integrative Cancer Research, is also a senior author of the paper.
*** It would take one billion nanoliter drops to fill 4 cups.
Abstract of Miniaturized neural system for chronic, local intracerebral drug delivery
Recent advances in medications for neurodegenerative disorders are expanding opportunities for improving the debilitating symptoms suffered by patients. Existing pharmacologic treatments, however, often rely on systemic drug administration, which result in broad drug distribution and consequent increased risk for toxicity. Given that many key neural circuitries have sub–cubic millimeter volumes and cell-specific characteristics, small-volume drug administration into affected brain areas with minimal diffusion and leakage is essential. We report the development of an implantable, remotely controllable, miniaturized neural drug delivery system permitting dynamic adjustment of therapy with pinpoint spatial accuracy. We demonstrate that this device can chemically modulate local neuronal activity in small (rodent) and large (nonhuman primate) animal models, while simultaneously allowing the recording of neural activity to enable feedback control.
