Monkeys learn to drive wheelchairs with their thoughts
March 3, 2016
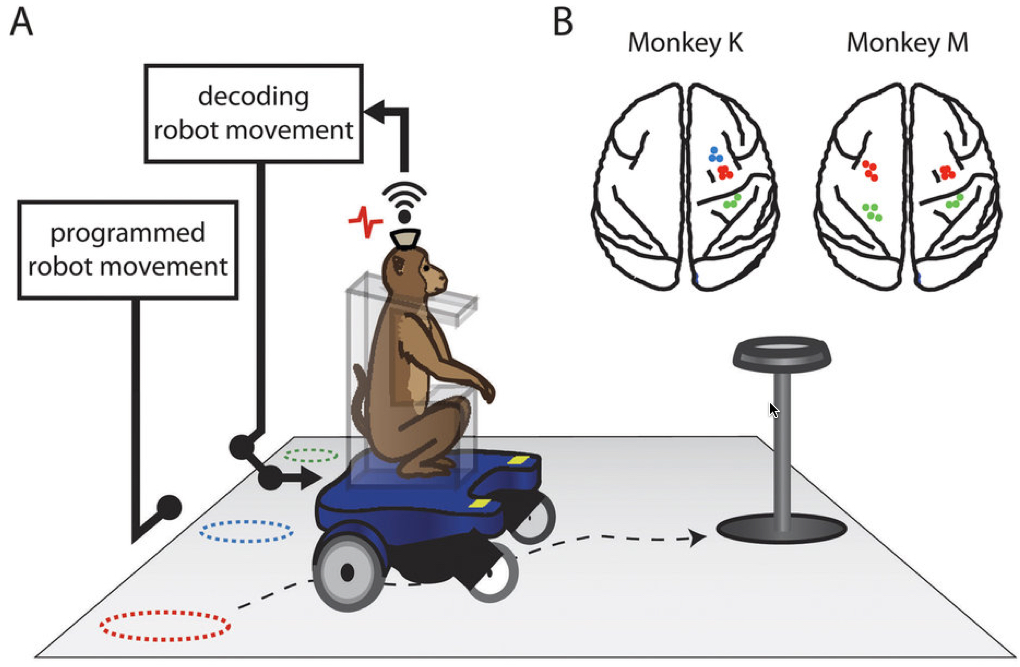
(A) The mobile robotic wheelchair, which seats a monkey, was moved from one of the three starting locations (dashed circles) to a grape dispenser as the wireless recording system recorded the spiking activities from the monkey’s brain along with the passive wheelchair movement, creating programmed movements. (B) Brain regions from which velocity or steering for the two monkeys was recorded. (credit: S. Rajangam et al./Scientific Reports)
Duke Health neuroscientists have developed a brain-machine interface (BMI) that allows monkeys to steer a robotic wheelchair with their thoughts.
The BMI uses signals from hundreds of neurons recorded simultaneously in two regions of the monkeys’ brains that are involved in movement and sensation. As the animals think about moving toward their goal — in this case, a bowl containing fresh grapes — computers translate their brain activity into real-time operation of the wheelchair.
The interface, described in the March 3 issue of the open-access online journal Scientific Reports, demonstrates the future potential for people with disabilities who have lost most muscle control and mobility due to quadriplegia or ALS, said senior author Miguel Nicolelis, M.D., Ph.D., co-director for the Duke Center for Neuroengineering.
Nature Publishing Group | Incredible moment MONKEY drives wheelchair using brain power
“In some severely disabled people, even blinking is not possible,” Nicolelis said. “For them, using a wheelchair or device controlled by noninvasive measures like an EEG (a device that monitors brain waves through electrodes on the scalp) may not be sufficient. We show clearly that if you have intracranial implants, you get better control of a wheelchair than with noninvasive devices.”
Recording brain activity
Scientists began the experiments in 2012, implanting hundreds of hair-thin microfilaments in the premotor and somatosensory regions of the brains of two rhesus macaques. They trained the animals by passively navigating the chair toward the bowl containing grapes. During this training phase, the scientists recorded the primates’ large-scale electrical brain activity. The researchers then programmed a computer system to translate these recorder brain signals into digital motor commands that later controlled the movements of the wheelchair.
A computer in the lab of Miguel Nicolelis, M.D., Ph.D., monitors brain signals from a rhesus macaque (credit: Shawn Rocco/ Duke Health)
This process is similar to using recorded brain patterns of experienced pilots to train novice pilots (see “Now you can learn to fly a plane from expert-pilot brainwave patterns“), except that in this case, the monkey’s own brain activity was recorded. As the monkeys learned to control the wheelchair just by thinking, they became more efficient at navigating toward the grapes and completed the trials faster, Nicolelis said.
The primates’ brain signals showed signs they were estimating their distance to the bowl of grapes. “This was not a signal that was present in the beginning of the training, but something that emerged as an effect of the monkeys becoming proficient in this task,” Nicolelis said. “This was a surprise. It demonstrates the brain’s enormous flexibility to assimilate a device, in this case a wheelchair, and that device’s spatial relationships to the surrounding world.”
Human version next
The trials measured the activity of nearly 300 neurons in each of the two monkeys. The team now hopes to expand the experiment by recording more neuronal signals to continue to increase the accuracy and fidelity of the primate BMI before seeking trials for an implanted device in humans, he said.
“BMIs can lead to partial neurological recovery or even augment brain function because their chronic and continuous use may trigger widespread cortical plasticity and the emergence of new cortical representations,” the reseachers note in the paper.
The National Institutes of Health funded this study. The Itau Bank of Brazil provided research support to the study as part of the Walk Again Project, an international non-profit consortium aimed at developing new assistive technologies for severely paralyzed patients.
Abstract of iWireless Cortical Brain-Machine Interface for Whole-Body Navigation in Primates
Several groups have developed brain-machine-interfaces (BMIs) that allow primates to use cortical activity to control artificial limbs. Yet, it remains unknown whether cortical ensembles could represent the kinematics of whole-body navigation and be used to operate a BMI that moves a wheelchair continuously in space. Here we show that rhesus monkeys can learn to navigate a robotic wheelchair, using their cortical activity as the main control signal. Two monkeys were chronically implanted with multichannel microelectrode arrays that allowed wireless recordings from ensembles of premotor and sensorimotor cortical neurons. Initially, while monkeys remained seated in the robotic wheelchair, passive navigation was employed to train a linear decoder to extract 2D wheelchair kinematics from cortical activity. Next, monkeys employed the wireless BMI to translate their cortical activity into the robotic wheelchair’s translational and rotational velocities. Over time, monkeys improved their ability to navigate the wheelchair toward the location of a grape reward. The navigation was enacted by populations of cortical neurons tuned to whole-body displacement. During practice with the apparatus, we also noticed the presence of a cortical representation of the distance to reward location. These results demonstrate that intracranial BMIs could restore whole-body mobility to severely paralyzed patients in the future.
