Nanoprobes for deep-tissue optical imaging of proteins in neurons
April 1, 2014
Berkeley Lab researchers have created nanoparticles that can be safely used to image single proteins in a cell without disrupting the protein’s activity (credit: Andrew Mueller)
In a potential breakthrough in brain-tissue imaging, Lawrence Berkeley National Laboratory (Berkeley Lab) researchers have developed ultra-tiny (sub-10-nanometers), ultra-bright nanoprobes for single-molecule deep-tissue optical imaging of proteins in neurons in the brain and other tissues.
Scientists often study proteins within cells by labeling them with light-emitting probes, but finding probes that are bright enough for imaging — but not so large as to disrupt the protein’s function — has been a challenge.
Fluorescent organic dye molecules and semiconductor quantum dots meet the size requirements but impose other limitations.
“Organic dyes and quantum dots will blink, meaning they randomly turn on and off, which is quite problematic for single-molecule imaging, and will photobleach and turn-off permanently, usually after less than 10 seconds under most imaging conditions,” says James Schuck, who directs the Berkeley Lab’s Molecular Foundry’s Imaging and Manipulation of Nanostructures Facility.
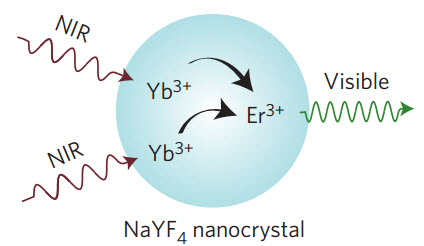
Upconversion of near-infrared light to visible light (credit: Daniel J. Gargas et al./Nature Nanotechnology)
So five years ago, researchers at the Molecular Foundry synthesized and imaged single nanoparticles from nanocrystals of sodium yttrium fluoride (NaYF4) doped with trace amounts of the lanthanide elements ytterbium and erbium.
These nanoparticles are able to upconvert near-infrared photons into green or red visible light (for use in microscopes), and their photostability makes them potentially ideal luminescent probes for single-molecule imaging. (Upconversion is the process by which a molecule absorbs two or more photons at a lower energy and emits them at higher energies.)
The researchers have now found that they can also reduce the size of these nanoparticles to as small as 4.8 nanometers without loss of brightness by raising erbium concentration and reducing ytterbium concentration.
This research was published in Nature Nanotechnology and supported by the DOE Office of Science.
Abstract of Nature Nanotechnology paper
Imaging at the single-molecule level reveals heterogeneities that are lost in ensemble imaging experiments, but an ongoing challenge is the development of luminescent probes with the photostability, brightness and continuous emission necessary for single-molecule microscopy. Lanthanide-doped upconverting nanoparticles overcome problems of photostability and continuous emission and their upconverted emission can be excited with near-infrared light at powers orders of magnitude lower than those required for conventional multiphoton probes. However, the brightness of upconverting nanoparticles has been limited by open questions about energy transfer and relaxation within individual nanocrystals and unavoidable tradeoffs between brightness and size. Here, we develop upconverting nanoparticles under 10 nm in diameter that are over an order of magnitude brighter under single-particle imaging conditions than existing compositions, allowing us to visualize single upconverting nanoparticles as small (d = 4.8 nm) as fluorescent proteins. We use advanced single-particle characterization and theoretical modelling to find that surface effects become critical at diameters under 20 nm and that the fluences used in single-molecule imaging change the dominant determinants of nanocrystal brightness. These results demonstrate that factors known to increase brightness in bulk experiments lose importance at higher excitation powers and that, paradoxically, the brightest probes under single-molecule excitation are barely luminescent at the ensemble level.
