Nanosheet catalyst sustainably splits hydrogen from water
May 10, 2012
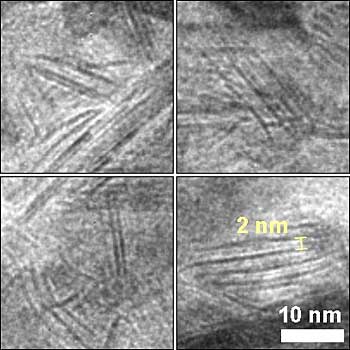
Transmission electron microscope image of unexpected nanosheet structure of a nickel-molybdenum-nitride catalyst, seen here as dark, straight lines (credit: Brookhaven National Laboratory)
A new electrocatalyst that generates hydrogen gas from water cleanly and with affordable materials has been developed by scientists at the U.S. Department of Energy’s (DOE) Brookhaven National Laboratory.
Traditional methods of producing pure hydrogen face significant challenges by either releasing harmful carbon dioxide into the atmosphere or requiring rare and expensive chemical elements such as platinum.
The novel form of catalytic nickel-molybdenum-nitride surprised scientists with its high-performing nanosheet structure, introducing a new model for effective hydrogen catalysis.
The electrolysis of water — splitting water (H2O) into oxygen (O2) and hydrogen (H2) — requires external electricity and an efficient catalyst to break chemical bonds while shifting around protons and electrons.
Nanosheets
Platinum is the gold standard for electrocatalysis, but with rapidly rising costs — already hovering around $50,000 per kilogram — and limited supplies, platinum and other noble metals discourage widespread investment.
In contrast, the principal metals in the new compound developed by the Brookhaven team are both abundant and cheap: $20 per kilogram for nickel and $32 per kilogram for molybdenum. Combined, that’s 1000 times less expensive than platinum.
By subjecting the compound to a high-temperature ammonia environment, they infused the nickel-molybdenum with nitrogen, which expanded the lattice of nickel-molybdenum, increased its electron density, made an electronic structure approaching that of noble metals, and prevented corrosion. But it also transformed the particles into unexpected two-dimensional nanosheets.
The nanosheet structures offer highly accessible reactive sites — consider the surface area difference between bed sheets laid out flat and those crumpled up into balls — and therefore more reaction potential.
Hydrogen future
The new catalyst performs nearly as well as platinum, achieving electrocatalytic activity and stability unmatched by any other non-noble metal compounds. “The production process is both simple and scalable,” Muckerman said, “making nickel-molybdenum-nitride appropriate for wide industrial applications.”
While this catalyst does not represent a complete solution to the challenge of creating affordable hydrogen gas, it does offer a cost major reduction.
Ref.: Dr. Wei-Fu Chen et al., Hydrogen-Evolution Catalysts Based on Non-Nobel Metal Nickel–Molybdenum Nitride Nanosheets, Angewandte Chemie, 2012, DOI: 10.1002/anie.201200699
