Nanowire solar cells reduce cost
September 1, 2011

Creating a core/shell nanowire solar cell, starting from left with a CdS nanowire (green) that is dipped in CuCl, where cation exchange reaction creates a Cu2S shell coating (brown). Metal contacts are then deposited on the CdS core and Cu2S shell (credit: Yang, et. al)
A team of researchers with the U.S. Department of Energy (DOE)’s Lawrence Berkeley National Laboratory (Berkeley Lab) has demonstrated that solar cells can be made from inexpensive elements using low-cost, less-energy-intensive processing chemistry — increasing the possibility of efficiently and cost-competitively converting sunlight into electricity.
The researchers developed a technique for fabricating core/shell nanowire solar cells using the semiconductors cadmium sulfide for the core and copper sulfide for the shell. These inexpensive and easy-to-make nanowire solar cells boasted open-circuit voltage and fill factor values superior to conventional planar solar cells. Together, the open-circuit voltage and fill factor determine the maximum energy that a solar cell can produce. In addition, the new nanowires also demonstrated an energy conversion efficiency of 5.4 percent, which is comparable to planar solar cells.
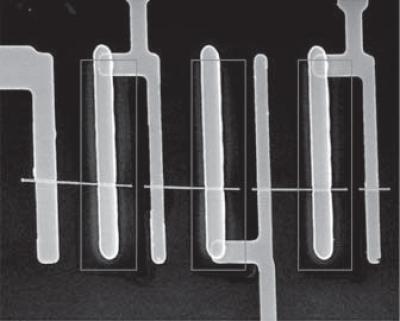
This scanning electron microscopy image shows three solar cells in series on a single nanowire with the core (credit: Yang, et. al)
At the heart of all solar cells are two separate layers of material, one with an abundance of electrons that function as a negative pole, and one with an abundance of electron holes (positively-charged energy spaces) that function as a positive pole.
When photons from the sun are absorbed, their energy is used to create electron-hole pairs, which are then separated at the p-n junction — the interface between the two layers —and collected as electricity.
The researchers developed a relatively inexpensive way to replace the planar p-n junctions of conventional solar cells with a radial p-n junction, in which a layer of n-type silicon formed a shell around a p-type silicon nanowire core. This geometry effectively turned each individual nanowire into a photovoltaic cell and greatly improved the light-trapping capabilities of silicon-based photovoltaic thin films.
They applied this strategy to the fabrication of core/shell nanowires using cadmium sulfide and copper sulfide, but this time using solution chemistry. These core/shell nanowires were prepared using a solution-based cation (negative ion) exchange reaction. The cadmium sulfide nanowires were then dipped into a solution of copper chloride at a temperature of 50 degrees Celsius and kept there for 5 to 10 seconds. The cation exchange reaction converted the surface layer of the cadmium sulfide into a copper sulfide shell.
Ref.: Jinyao Tang, et al., Solution-processed core–shell nanowires for efficient photovoltaic cells, Nature Nanotechnology, 2011; [DOI: 10.1038/nnano.2011.139]
