Natural plant compound prevents Alzheimer’s disease in mice
January 30, 2014
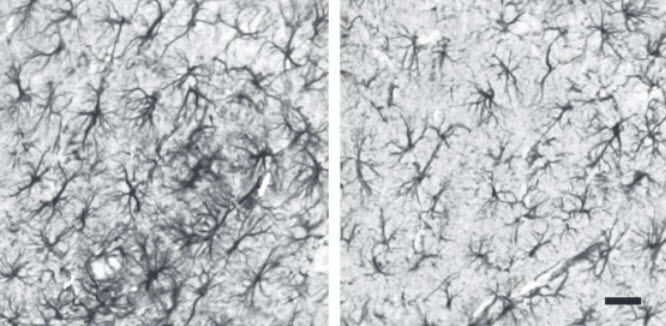
Fisetin reduces (right) astrocytic reactivity in the brains of Alzheimer’s disease mice. Scale bar: 10 microns. (Credit: Antonio Currais et al./Aging Cell)
A daily dose of fisetin, an antioxidant chemical that’s found in fruits and vegetables from strawberries to cucumbers, appears to stop memory loss that accompanies Alzheimer’s disease in mice, scientists at the Salk Institute for Biological Studies have discovered.
In experiments on mice that normally develop Alzheimer’s symptoms less than a year after birth, a daily dose of the compound prevented the progressive memory and learning impairments.
The drug, however, did not alter the formation of amyloid plaques in the brain, accumulations of proteins that are commonly blamed for Alzheimer’s disease. The new finding suggests a way to treat Alzheimer’s symptoms independently of targeting amyloid plaques.
“We had already shown that in normal animals, fisetin can improve memory,” says Pamela Maher, a senior staff scientist in Salk’s Cellular Neurobiology Laboratory who led the new study. “What we showed here is that it also can have an effect on animals prone to Alzheimer’s.”
More than a decade ago, Maher discovered that fisetin helps protect neurons in the brain from the effects of aging. She and her colleagues have since — in both isolated cell cultures and mouse studies — probed how the compound has both antioxidant and anti-inflammatory effects on cells in the brain. Most recently, they found that fisetin turns on a cellular pathway known to be involved in memory.
“What we realized is that fisetin has a number of properties that we thought might be beneficial when it comes to Alzheimer’s,” says Maher.
The experiments
So Maher — who works with Dave Schubert, the head of the Cellular Neurobiology Lab — turned to a strain of mice that have mutations in two genes linked to Alzheimer’s disease. The researchers took a subset of these mice and, when they were only three months old, began adding fisetin to their food.
As the mice aged, the researchers tested their memory and learning skills with water mazes. By nine months of age, mice that hadn’t received fisetin began performing more poorly in the mazes. Mice that had gotten a daily dose of the compound, however, performed as well as normal mice, at both nine months and a year old.
“Even as the disease would have been progressing, the fisetin was able to continue preventing symptoms,” Maher says.
In collaboration with scientists at the University of California, San Diego, Maher’s team next tested the levels of different molecules in the brains of mice that had received doses of fisetin and those that hadn’t. In mice with Alzheimer’s symptoms, they found, pathways involved in cellular inflammation were turned on. In the animals that had taken fisetin, those pathways were dampened and anti-inflammatory molecules were present instead.
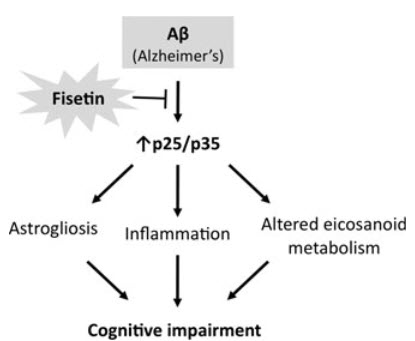
One protein in particular — known as p35 — was blocked from being cleaved into a shorter version when fisetin was taken. The shortened version of p35 is known to turn on and off many other molecular pathways. The results were published December 17, 2013, in the journal Aging Cell.
Next steps
Next, Maher’s team hopes to understand more of the molecular details on how fisetin affects memory, including whether there are targets other than p35.
“It may be that compounds like this that have more than one target are most effective at treating Alzheimer’s disease,” says Maher, “because it’s a complex disease where there are a lot of things going wrong.”
They also aim to develop new studies to look at how the timing of fisetin doses affect its influence on Alzheimer’s.
“The model that we used here was a preventive model,” explains Maher. “We started the mice on the drugs before they had any memory loss. But obviously human patients don’t go to the doctor until they are already having memory problems.” So the next step in moving the discovery toward the clinic, she says, is to test whether fisetin can reverse declines in memory once they have already appeared.
The work was supported by grants from the Alzheimer’s Association, Paul Slavik, the National Institutes of Health, the Alzheimer’s Drug Discovery Foundation, and the George E. Hewitt Foundation.
Abstract of Aging Cell paper
Alzheimer’s disease (AD) is the most common type of dementia. It is the only one of the top ten causes of death in the USA for which prevention strategies have not been developed. Although AD has traditionally been associated with the deposition of amyloid β plaques and tau tangles, it is becoming increasingly clear that it involves disruptions in multiple cellular systems. Therefore, it is unlikely that hitting a single target will result in significant benefits to patients with AD. An alternative approach is to identify molecules that have multiple biological activities that are relevant to the disease. Fisetin is a small, orally active molecule which can act on many of the target pathways implicated in AD. We show here that oral administration of fisetin to APPswe/PS1dE9 double transgenic AD mice from 3 to 12 months of age prevents the development of learning and memory deficits. This correlates with an increase in ERK phosphorylation along with a decrease in protein carbonylation, a marker of oxidative stress. Importantly, fisetin also reduces the levels of the cyclin-dependent kinase 5 (Cdk5) activator p35 cleavage product, p25, in both control and AD brains. Elevated levels of p25 relative to p35 cause dysregulation of Cdk5 activity leading to neuroinflammation and neurodegeneration. These fisetin-dependent changes correlate with additional anti-inflammatory effects, including alterations in global eicosanoid synthesis, and the maintenance of markers of synaptic function in the AD mice. Together, these results suggest that fisetin may provide a new approach to the treatment of AD.