Wearable artificial kidney prototype successfully tested
June 14, 2016
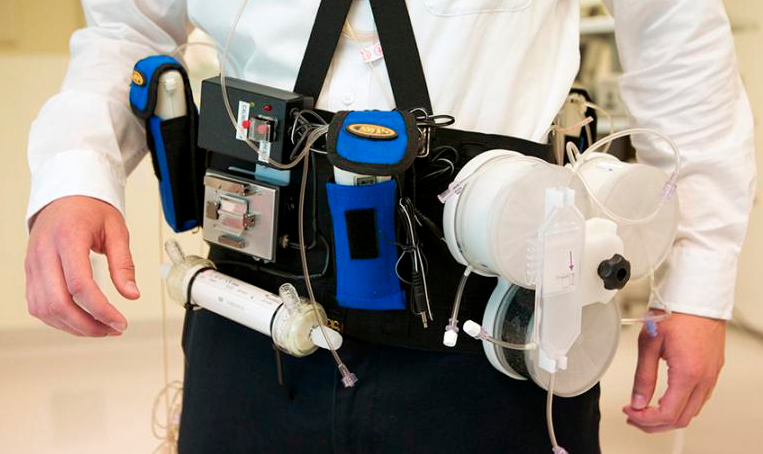
Working prototype of wearable artificial kidney developed by Victor Gura, MD, and his team (credit: Stephen Brashear/University of Washington)
An FDA-approved exploratory clinical trial of a prototype wearable artificial kidney (WAK) — a miniaturized, wearable hemodialysis machine — at the University of Washington Medical Center in Seattle has been completed, the researchers reported June 2 in an open-access paper in JCI Insight.
The seven patients enrolled in the study reported “significantly greater treatment satisfaction during the WAK treatment period compared with ratings of care during periods of conventional in-center hemodialysis treatment,” according to the researchers.
“During the study, hemodynamic parameters remained stable, ultrafiltration was achieved as intended, and there were no unexpected adverse treatment effects.” The study was led by the device inventor, Victor Gura, M.D., of Cedars-Sinai Medical Center in Los Angeles and Blood Purification Technologies Inc.
The trial was stopped after the seventh subject due to device-related technical problems, including excessive carbon dioxide bubbles in the dialysate circuit and variable blood and dialysate flows, which the scientists plan to fix.
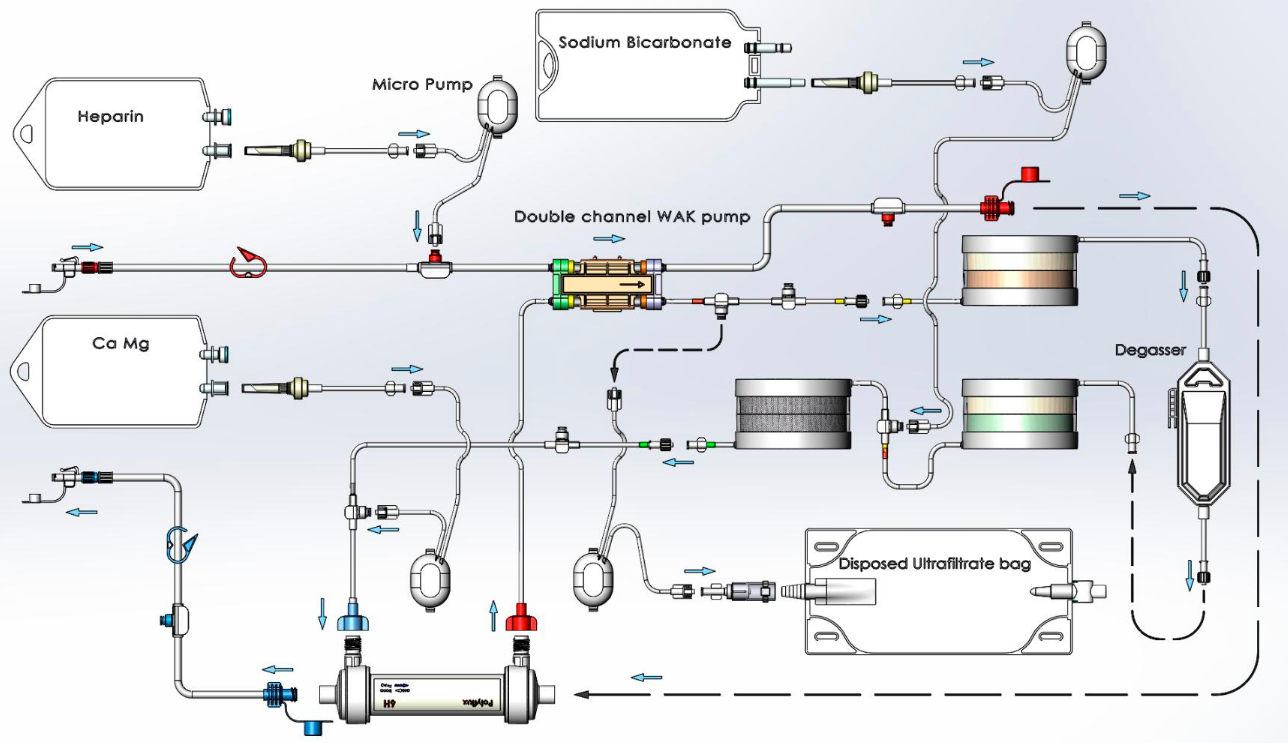
Detailed schematic flow diagram of wearable artificial kidney blood and dialysate circuits. ZP: zirconium phosphate; HZO: hydrous zirconium oxide; CO2EF: semipermeable degassing bubble removal mechanism. (credit: Victor Gura et al./JCI Insight)
More than 2 million people worldwide experience end-stage renal disease (ESRD), which is currently treated with hemodialysis therapies that require patients to adhere to restrictive dietary and fluid intake limitations and are associated with a high pill burden, according to the researchers. Adjusted rates of all-cause mortality are up to 8 times greater for dialysis patients compared with age-matched individuals in the general population, they note.
The WAK is designed to be worn and used by patients for up to 24 hours per day. The hope is that treatment can be administered at the patients’ homes either by the patients themselves or caretakers. Being able to be ambulatory while undergoing dialysis, if further proven in additional studies, “would liberate patients from the need to be tethered to a stationary machine during dialysis treatments,” according to the researchers.

(credit: Blood Purification Technologies Inc.)
The researchers caution that “long-term safety of continuous treatment with the WAK has not been established yet. Longer-term studies treating patients in the outpatient and home environment are necessary to address safety issues during ambulation and the home operation of the device by patients and to incorporate additional human factor elements.”
To learn more or donate, contact Wearable Artifical Kidney Foundation, which funded the study along with Blood Purification Technologies Inc.
UWMedicineHealth | Wearable Artificial Kidney: first U.S. clinical trial
