Why your immune system may control your social behavior
July 15, 2016
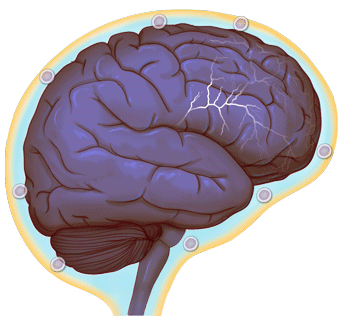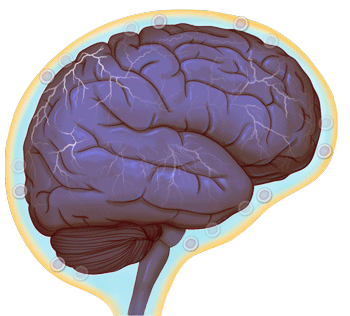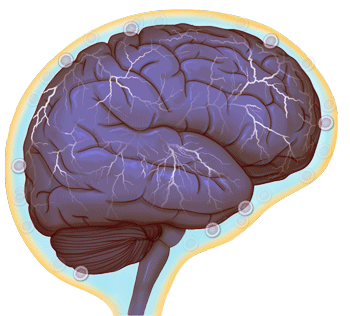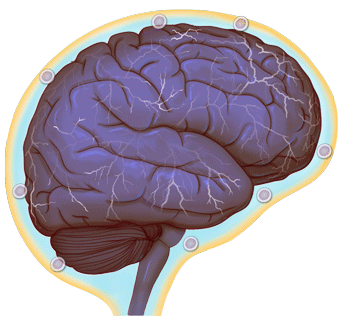
Normal brain activity (credit: University of Virginia Health System)
In a discovery that raises fundamental questions about human behavior, researchers at the University of Virginia School of Medicine have found that the immune system directly affects — and even controls — our social behavior, such as our desire to interact with others. That finding could have significant implications for neurological diseases such as autism-spectrum disorders and schizophrenia, the researchers suggest.
“The brain and the adaptive immune system were thought to be isolated from each other, and any immune activity in the brain was perceived as sign of a pathology. And now, not only are we showing that they are closely interacting, but some of our behavior traits might have evolved because of our immune response to pathogens,” explained Jonathan Kipnis, chair of UVA’s Department of Neuroscience.
“It’s crazy, but maybe we are just multicellular battlefields for two ancient forces: pathogens and the immune system. Part of our personality may actually be dictated by the immune system.”
Evolutionary forces linking brains and pathogens
KurzweilAI has cited supporting evidence for that idea. For example, permanent stress may affect immune cells in the brain, leading to mental disorders and protective immune microglia cells also have direct involvement in creating the cellular networks at the core of brain behavior.
Last year, Kipnis, the director of UVA’s Center for Brain Immunology and Glia, and his team discovered that meningeal membranes (covering the brain and spinal cord) in the brain directly link the brain with the lymphatic system. That overturned decades of textbook teaching that the brain lacks a direct connection to the immune system.
Now, the researchers suggest, the relationship between people and pathogens could have directly affected the development of our social behavior. Social behavior (which is necessary for the survival of the species) allows pathogens to spread, so our immune systems may have developed to protect us from the diseases that accompany those interactions.
Specifically, the UVA researchers have now shown that a specific immune molecule, interferon gamma, seems to be critical for social behavior, and that a variety of creatures, such as flies, zebrafish, mice and rats, activate interferon gamma responses (as protection) when they are social.
Normally, this molecule is produced by the immune system in response to bacteria, viruses or parasites. But blocking the molecule in mice using genetic modification also made regions of the brain hyperactive, causing the mice to become less social. Restoring the molecule restored the brain connectivity and behavior to normal.
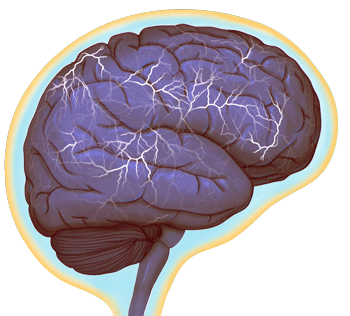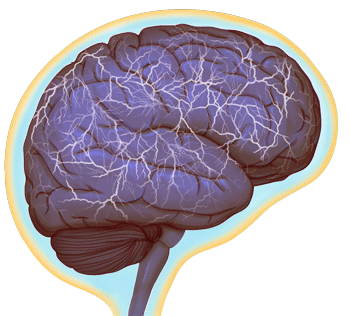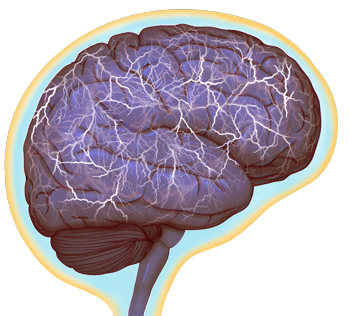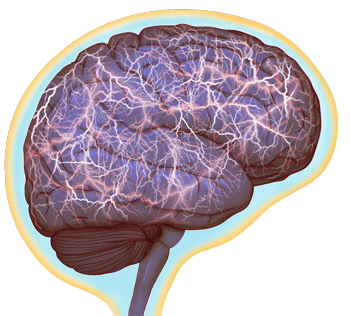
A hyperactive brain, triggered by a blocked immune system, may lead to less-social behavior (credit: University of Virginia Health System)
In a Nature paper outlining their findings, the researchers note the immune molecule plays a “profound role in maintaining proper social function.”
“It’s extremely critical for an organism to be social for the survival of the species. It’s important for foraging, sexual reproduction, gathering, hunting,” said Anthony J. Filiano, Hartwell postdoctoral fellow in the Kipnis lab and lead author of the study. “The hypothesis is that when organisms come together, you have a higher propensity to spread infection — you need to be social, but [in doing so] you have a higher chance of spreading pathogens.” Which explains why “interferon gamma, in evolution, has been used as a more efficient way to both boost social behavior while boosting an anti-pathogen response.”
Immune-system failure leads to social deficits
The researchers explain that a malfunctioning immune system may be responsible for “social deficits in numerous neurological and psychiatric disorders.” But exactly what this might mean for autism and other specific conditions requires further investigation.
It is unlikely that any one molecule will be responsible for disease or the key to a cure. The researchers believe that the causes are likely to be much more complex. But the discovery that the immune system — and possibly pathogens, by extension — can control our interactions raises many exciting avenues for scientists to explore, both in terms of battling neurological disorders and understanding human behavior.
Kipnis and his team worked closely with UVA’s Department of Pharmacology and with Vladimir Litvak’s research group at the University of Massachusetts Medical School. Litvak’s team developed a computational approach to investigate the complex dialogue between immune signaling and brain function in health and disease.
“Using this approach we predicted a role for interferon gamma, an important cytokine secreted by T lymphocytes, in promoting social brain functions,” Litvak said. “Our findings contribute to a deeper understanding of social dysfunction in neurological disorders, such as autism and schizophrenia, and may open new avenues for therapeutic approaches.”
The work was supported by NIH grants and the Hartwell Foundation.
Medicine Virginia | Shocking New Role Found for the Immune System: Controlling Social Interactions
Abstract of Unexpected role of interferon-γ in regulating neuronal connectivity and social behaviour
Immune dysfunction is commonly associated with several neurological and mental disorders. Although the mechanisms by which peripheral immunity may influence neuronal function are largely unknown, recent findings implicate meningeal immunity influencing behaviour, such as spatial learning and memory1. Here we show that meningeal immunity is also critical for social behaviour; mice deficient in adaptive immunity exhibit social deficits and hyper-connectivity of fronto-cortical brain regions. Associations between rodent transcriptomes from brain and cellular transcriptomes in response to T-cell-derived cytokines suggest a strong interaction between social behaviour and interferon-γ (IFN-γ)-driven responses. Concordantly, we demonstrate that inhibitory neurons respond to IFN-γ and increase GABAergic (γ-aminobutyric-acid) currents in projection neurons, suggesting that IFN-γ is a molecular link between meningeal immunity and neural circuits recruited for social behaviour. Meta-analysis of the transcriptomes of a range of organisms reveals that rodents, fish, and flies elevate IFN-γ/JAK-STAT-dependent gene signatures in a social context, suggesting that the IFN-γ signalling pathway could mediate a co-evolutionary link between social/aggregation behaviour and an efficient anti-pathogen response. This study implicates adaptive immune dysfunction, in particular IFN-γ, in disorders characterized by social dysfunction and suggests a co-evolutionary link between social behaviour and an anti-pathogen immune response driven by IFN-γ signalling.
